A New Method for Synthesizing Poly(thioether)s at J. Am. Chem. Soc., Developed by Prof. Xinghong Zhang et. al, The Group of Department of Polymer Science and Engineering, Zhejiang University
Time: 2019-04-11 visit times :52
【Research Background】Aliphatic poly(thioether) is a functional polymeric material with potential applications in optical materials, energy storage, metal ion detection, and rechargeable batteries. However, the synthetic method to aliphatic poly(thioether)s still remains a huge challenge. The synthesis of aliphatic poly(thioether)s which have been reported so far is mainly achieved by two means, ring-opening polymerization of episulfide and click reaction of thiol group with double bond or triple bond. However, the polymerizable monomers are more difficult to obtain on a large scale and are relatively expensive. And these monomers are active and easy to oxidize, and require special storage conditions.
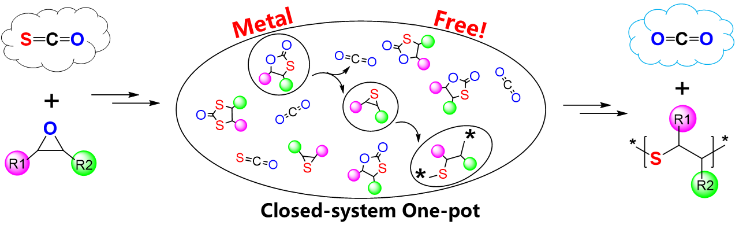
Figure 1. One-pot closed-system synthesis of poly(thioether)s (killing two birds with one stone method).
【Main Content】Recently, Xinghong Zhang's group used sulfur-containing mono-carbon monomer (COS) and epoxide as monomers to realize the one-pot synthesis of poly(thioether) in a closed system. All catalysts are common organic bases. For a dozen common epoxy compounds, the method shows good effects, that is, poly(thioether)s with different structures and properties are prepared. The glass transition temperature ranges from -58 to 53 oC based on the side groups, and the high refractive index is as high as 1.64; the obtained poly(thioether) has a mercapto group at one end that has good reactivity. These structural features give this type of polymer a broad application space.
There are two facts inspiring us to explore this work: First, the ring-openning polymerization of five-membered cyclic carbonate is often at high temperatures, and the entropy effect inevitably leads to the formation of polyether segments; the other is the oxygen-sulfur exchange reaction discovered by our group. The reaction (Macromolecules, 2008, 41, 1587), in the system in which sulfur-containing C1 is copolymerized with an epoxide, generates some poly(thioether) segments mixed in the final polymers. These prompted us to try to use the oxygen-sulfur exchange reaction to prepare poly(thioether)s. Further theoretical calculations combined with experimental results identify the mechanism process that is shown in Figure 2. First, the coupling reaction between COS and epoxide occurs to form the five-membered cyclic monothiocarbonates (and a small amount of cyclic dithiocarbonates); the sulfur anion formed by the organic base and the cyclic thiocarbonate in the initiation stage, selectively attack the carbonate-based position of the ring; the subsequent ring-opening, desulfonation of thiopropane and decarboxylation reactions complete chain growth to form a polysulfide, wherein the sulfur anion can selectively attack the methylene position of the thiopropane. Thus, the oxygen-sulfur atom exchange reaction is successfully utilized.

Figure 2. Reaction mechanism proposed by theoretical calculation (regioselective ring-opening polymerization involving five-membered ring and epithiopropane).
This work provides a new option for the large-scale synthesis of aliphatic poly(thioether)s containing terminal sulfhydryl groups. Although COS can come from cheap raw materials such as CO and S, or enrichment and recovery, they are all energy-intensive ways. Therefore, the difficulty in the future lies in how to obtain low-cost COS in the industry.
The corresponding author is Professor Xinghong Zhang, from the Department of Polymer Science and Engineering, Zhejiang University. Special thanks to the co-author, Prof. Xin Hong and Dr. Tiancheng Zhu, from the Department of Chemistry, Zhejiang University , for their extensive work in theoretical calculations. Special thanks to the first author, Dr. Chengjian Zhang, for his keen observation and hard work.
Related Links:
Poly(thioether)s from Closed-System One-Pot Reaction of Carbonyl Sulfide and Epoxides by Organic Bases
Cheng-Jian Zhang, Tian-Cheng Zhu, Xiao-Han Cao, Xin Hong, and Xing-Hong Zhang*
J. Am. Chem. Soc. 2019, 141, 5490−5496
https://pubs.acs.org.ccindex.cn/doi/abs/10.1021/jacs.9b00544
Zhe Da Road 38, Hangzhou 310027, China
Tel : 86-571-87951308
Fax : 86-571-87951592
Email : ciciliu33@zju.edu.cn